Pt/porous nanorods of ceria as efficient high temperature catalysts with remarkable catalytic stability for carbon dioxide reforming of methane†
Abstract
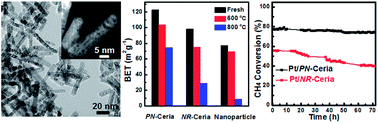
Porous nanorods of ceria (PN-ceria) with a large oxygen storage capacity and a high concentration of oxygen vacancies exhibited better thermal stability compared to other nanocerias. No obvious structural deconstruction of PN-ceria was observed when the material was annealed at 800 °C. As the support of metal nanocatalysts, Pt exhibited a high dispersion on PN-ceria and presented a high activity and catalytic stability for the carbon dioxide reforming of methane (CRM) reaction at a high temperature of 800 °C. The catalytic activity of Pt/PN-ceria catalysts in terms of methane conversion only slightly decreased from 77.3% to 74.2% with a slow carbon deposition rate of 0.1 mg coke gcat−1 h−1 after 72 hours of the CRM reaction at 800 °C. In contrast, a large decay in methane conversion (16.2%) was observed for Pt/nonporous nanorods of ceria under the same reaction conditions. The high and robust catalytic activity of Pt/PN-ceria catalysts can be ascribed to the unique physicochemical properties of PN-ceria, by preventing the sintering of Pt nanocatalysts into big particles and the formation of coke under rigid reaction conditions. Hence, more accessible active sites of Pt/PN-ceria catalysts are provided for the CRM. Besides, the high concentration of oxygen vacancies and fast mobility of the lattice oxygen of PN-ceria also benefited the catalytic activity, stability and anti-coke deposition.

 Please wait while we load your content...
Please wait while we load your content...