Fabrication of hybrid membranes by incorporating acid–base pair functionalized hollow mesoporous silica for enhanced proton conductivity†
Abstract
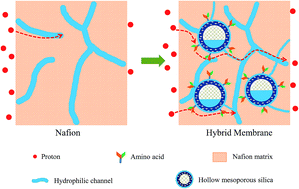
Hollow mesoporous silica microspheres with a uniform pore size and a large surface area are synthesized and functionalized by three kinds of amino acids with different acid–base pairs, including sulfonic acid–amino groups (HMS-Cys), phosphoric acid–amino groups (HMS-Phos) and carboxylic acid–amino groups (HMS-Asp). The incorporation of these microspheres into a Nafion matrix enhances the water uptake and adjusts the organic–inorganic interface and hydrophilic ionic domains inside the membranes. Microspheres with a hollow mesoporous structure endow the membranes with strong water retention ability. The proton conductivities of hybrid membranes are more than 8 times higher than that of recast Nafion at 40 °C and 20% RH after 90 min of testing. The acid–base pairs within the membranes work as proton donors and acceptors, and the retained water molecules are used as hydrogen-bonded bridges, which reduce the energy barrier for proton conduction. At the filler content of 4 wt%, the HMS-Cys embedded membrane shows the highest proton conductivity of 1.19 × 10−1 S cm−1 (30 °C, 100% RH). In addition, hybrid membranes incorporated with amino acid functionalized HMS show small reliance on humidity. The proton conductivities of all the hybrid membranes are ∼5.29–11.1 times higher than that of the recast Nafion under 26.1% RH and 80 °C.

 Please wait while we load your content...
Please wait while we load your content...