Cation–anion double hydrolysis derived layered single metal hydroxide superstructures for boosted supercapacitive energy storage†
Abstract
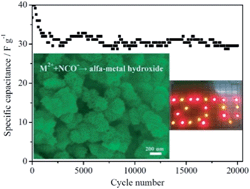
As promising battery-type electrode materials, layered single metal hydroxides (LSHs) including α-Ni(OH)2 and α-Co(OH)2 based hybrid supercapacitors exhibit larger operating voltages compared with those that are based on activated carbons and double-layer capacitance mechanisms. This study proposes a novel and facile room-temperature method to fabricate α-Ni(OH)2 and α-Co(OH)2 superstructures by using the double hydrolysis of Ni2+ or Co2+ and NCO− without the presence of any structure directing agent. Two dimensional sheet-like building blocks of the alpha-type metal hydroxide are assembled into various elegant morphologies including a 3D interconnected hierarchical assembly (3D-ICHA), sheet-on-sheet, sheet-on-rod and other nanostructures, which depends on the cation–anion mixing mode. Significantly, the 3D-ICHA α-Ni(OH)2 possesses an ultrahigh specific surface area (320.2 m2 g−1) and robust porous structure. An outstanding initial specific capacity (653.1 C g−1 at 1 A g−1 and 406 C g−1 at 20 A g−1) and an excellent cycling retention (86.2% in 20 000 cycles) were obtained for the 3D-ICHA α-Ni(OH)2, which stands out from most of the state-of-the-art α-Ni(OH)2 powder-based materials. A high-loading asymmetric capacitor with excessive activated carbon (∼10 mg 3D-ICHA α-Ni(OH)2vs. ∼60 mg activated carbon) is demonstrated and it works very steadily even after 20 000 charge/discharge cycles.

 Please wait while we load your content...
Please wait while we load your content...