Perovskite La0.6Sr0.4Cr1−xCoxO3−δ solid solutions for solar-thermochemical fuel production: strategies to lower the operation temperature†
Abstract
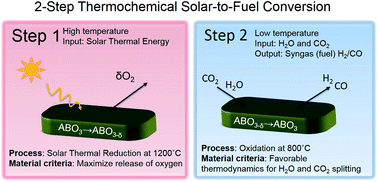
Storing abundant solar energy in synthetic fuels is key to ensure a sustainable energy future by replacing fossil fuels and reducing global warming emissions. Practical implementation of the solar-to-fuel technology is predicated on finding new materials with higher efficiency and lower operation temperature than state-of-the-art materials. We use criteria aimed for designing such efficient solar-to-fuel conversion materials in the perovskite system. Based on thermodynamic considerations, the first perovskite solute–solution series, La0.6Sr0.4Cr1−xCoxO3−δ, is investigated to gain fundamental understanding on the role of B-site cationic doping in water and CO2 splitting to produce synthetic fuel. Notably, all of the novel material compositions operate in a strongly lowered temperature regime of 800–1200 °C compared to state-of-the-art binary oxides in the field. We found an optimum in doping for fuel production performance, namely La0.6Sr0.4Cr0.8Co0.2O3−δ, which viably splits both CO2 and H2O. Based on thermogravimetric analysis, we show that the highest performing perovskite splits 25 times more CO2 compared to the current state-of-the-art material, ceria, for two-step thermochemical cycling at 800–1200 °C. No adverse formation of carbonates in a CO2 atmosphere or cation segregation was observed in near and long range structural investigations, which highlight the durability and potential of these solid solutions. These new perovskite compositions enable lowering of the standard solar-to-fuel reactor temperature by 300 °C. The lowered operating temperature has tremendous implications for solar-synthesized fuels in a reactor in terms of lowered heat loss, increased efficiency, and reactor materials.

 Please wait while we load your content...
Please wait while we load your content...