Bottom-up and top-down methods to improve catalytic reactivity for photocatalytic production of hydrogen peroxide using a Ru-complex and water oxidation catalysts†
Abstract
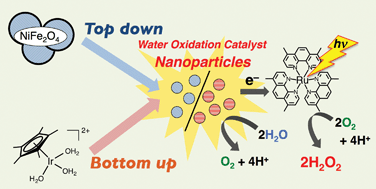
Hydrogen peroxide (H2O2) was produced from water and dioxygen using [RuII(Me2phen)3]2+ (Me2phen = 4,7-dimethyl-1,10-phenanthroline) as a photocatalyst and [Ir(Cp*)(H2O)3]2+ (Cp* = η5-pentamethylcyclopentadienyl) as a precursor of a water oxidation catalyst in the presence of Sc3+ in water under visible light irradiation. TEM and XPS measurements of residues in the resulting solution after the photocatalytic production of H2O2 indicated that [Ir(Cp*)(H2O)3]2+ was converted to Ir(OH)3 nanoparticles, which are actual catalytic species. The Ir(OH)3 nanoparticles produced in situ during the photocatalytic production of H2O2 were smaller in size than those prepared independently from hydrogen hexachloroiridiate (H2IrCl6), and exhibited higher catalytic reactivity for the photocatalytic production of H2O2. The photocatalytic production of H2O2 from water and dioxygen was also made possible when Ir(OH)3 nanoparticles were replaced by nickel ferrite (NiFe2O4) nanoparticles, which are composed of more earth abundant metals than iridium. The size of NiFe2O4 nanoparticles became smaller during the photocatalytic production of H2O2 to exhibit higher catalytic reactivity in the second run as compared with that in the first run. NiFe2O4 nanoparticles obtained by the treatment of NiFe2O4 in an aqueous solution of Sc3+ exhibited 33-times higher catalytic reactivity in H2O2-production rates than the as-prepared NiFe2O4. Thus, both the bottom-up method starting from a molecular complex [Ir(Cp*)(H2O)3]2+ and the top-down method starting from as-prepared NiFe2O4 to obtain nanoparticles with smaller size resulted in the improvement of the catalytic reactivity for the photocatalytic production of H2O2 from water and dioxygen.

 Please wait while we load your content...
Please wait while we load your content...