Improvement of hydrogen evolution under visible light over Zn1−2x(CuGa)xGa2S4 photocatalysts by synthesis utilizing a polymerizable complex method†
Abstract
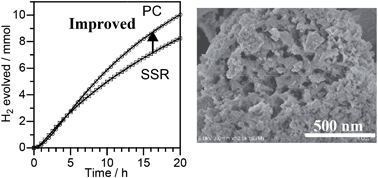
Single phase Zn1−2x(CuGa)xGa2S4 was successfully synthesized by a two-step route: synthesis of an oxide precursor by a polymerizable complex (PC) method followed by sulfurization under a H2S–Ar flow. The samples thus obtained were porous aggregates consisting of fine crystals with 50 nm size. Among the examined compositions, Zn0.4(CuGa)0.3Ga2S4 (x = 0.3) showed the highest activity for H2 evolution from an aqueous solution containing S2− and SO32− as electron donors under visible light irradiation. The apparent quantum yield of the optimized sample with deposition of 2 wt% of Rh was 19% at 420 nm. This value is higher than that of the sample synthesized by a solid state reaction (15%). Therefore, improvement of the photocatalytic performance of Zn0.4(CuGa)0.3Ga2S4 for H2 evolution has been achieved by using the two-step synthesis method including the preparation of an oxide precursor by the PC method.
- This article is part of the themed collection: Artificial Photosynthesis

 Please wait while we load your content...
Please wait while we load your content...