Incorporation of square-planar Pd2+ in fluorite CeO2: hydrothermal preparation, local structure, redox properties and stability†
Abstract
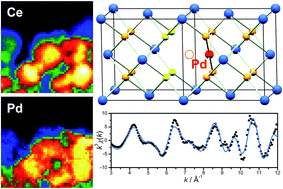
The direct hydrothermal crystallisation at 240 °C of Pd2+-containing ceria is investigated to study the extent to which precious metal dopants may be introduced into the cubic fluorite lattice. Samples of composition Ce1−xPdxO2−δ, where 0 ≤ x ≤ 0.15 can be produced in which Pd is included within the CeO2 structure to give a linear lattice expansion. Attempts to produce higher Pd2+-substitution result in the formation of PdO as a secondary phase. Ce and Pd were determined to be in the +4 and +2 oxidation states, respectively, by X-ray absorption near edge structure, suggesting oxide deficiency as the mechanism of charge balance. Extended X-ray absorption fine structure (EXAFS) analysis at the Pd K-edge reveals that Pd2+ has local square-planar coordination, as expected, and that a structural model can fitted in which the average fluorite structure is maintained, but with Pd2+ sitting in the square faces of oxide ions present in the local cubic geometry of Ce. This model, consistent with previous modelling studies, gives an excellent fit to the EXAFS spectra, and explains the observed lattice expansion. Transmission electron microscopy analysis shows that Pd is well dispersed in the nanocrystalline ceria particles, and in situ powder XRD shows that upon heating in air the samples remain stable up to 800 °C. H2-TPR shows that Pd-substitution leads to low temperature (<200 °C) reduction of the oxide, which increases in magnitude with increasing Pd-substitution. On prolonged heating, however, the Pd is lost from the ceria lattice to give dispersed Pd metal, suggesting an inherent instability of Pd-doped CeO2.
- This article is part of the themed collection: Highlighting materials research in the UK for energy and sustainability

 Please wait while we load your content...
Please wait while we load your content...