Pyrochlore electrocatalysts for efficient alkaline water electrolysis†
Abstract
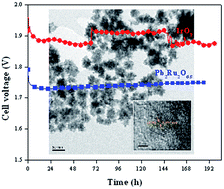
A series of electrically conducting metal oxides with the pyrochlore structure (A2B2O7−y, with A = Pb or Bi and B = Ru, Ir or Os) were synthesized via precipitation/crystallization in alkaline medium and/or via solid-state reaction. The electrocatalytic activity for the oxygen evolution reaction (OER) in 0.1 M KOH was studied using a rotating disk electrode. Lead and bismuth ruthenate pyrochlores showed significantly lower overpotentials for the OER than the state-of-the-art IrO2 catalyst. Specific activities (at 1.5 V vs. RHE) of 3.0 ± 0.2 A m−2, 1.3 ± 0.2 A m−2 and 0.06 ± 0.01 A m−2 were obtained for Pb2Ru2O6.5, Bi2.4Ru1.6O7, and IrO2 respectively. Specific activities for iridate-based pyrochlores (0.3–0.5 A m−2) were 5–10 times lower than those for ruthenate-based pyrochlores. Lead osmate pyrochlore showed the lowest OER activity among all the pyrochlores evaluated, with a specific activity of 0.10 ± 0.07 A m−2. It is proposed that the reaction path for the OER involves several oxygen intermediate species (–O2−, –OOH, –OO2−, –OH) bonded to the B-site (Ru, Ir or Os) in the pyrochlore, and that the catalytic activity depends on the bonding strength between the B-cation site and the oxygen species. This hypothesis was supported by the fact that OER activity correlated with Ru concentration in lead-rich lead ruthenate pyrochlores. The decrease of the specific OER activity depended on the occupancy of the 3d orbitals and on the period in the periodic table occupied by the B-cation. The OER activity decreased for pyrochlores with B cations having more d electrons than Ru, and when the B cation occupied period 6. The observed trend in activity was similar to that observed for the oxygen reduction reaction on transition metals, and was related to the strength of the bonding between the adsorbed oxygen species and the B-cation. The exceptional OER activity and stability of lead ruthenate pyrochlore catalysts were evaluated in a solid-state alkaline water electrolyzer. The overpotentials obtained were 0.1–0.2 V lower than for IrO2 and the performance was stable for at least 200 h.
- This article is part of the themed collection: Interdisciplinary Symposium on Materials Chemistry 2014

 Please wait while we load your content...
Please wait while we load your content...