Ab initio study of doping effects on LiMnO2 and Li2MnO3 cathode materials for Li-ion batteries†
Abstract
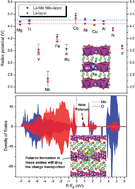
For the over-lithiated-oxides (OLOs), a composite of layered Li2MnO3 and LiMO2 (M = Mn, Co, Ni), the Li2MnO3 part is not stable after the 1st charge–discharge cycle and partly transforms into layered LiMnO2, which in practice indicates that the phase used is actually a mixture of both Li2MnO3 and LiMnO2. In the present work, the influence of 10 cationic (Mg, Ti, V, Nb, Fe, Ru, Co, Ni, Cu, and Al) and 2 anionic (N and F) dopants on the phase stability, redox potential, ionic and electronic conductivity of both Li2MnO3 and LiMnO2 is investigated in detail using density functional theory. The calculations show that all the cationic dopants and F can be thermodynamically stable in the layered structures. The redox potential of both oxides is quite sensitive to some of the dopants, like V, Nb, and Ru, due to the appearance of gap states introduced by those dopants. The Jahn–Teller effect has a strong influence on the Li vacancy diffusion behavior in both LiMnO2 and its doped phases. Li vacancy diffusion behavior in Li2MnO3, including both interlayer and intralayer pathways, is relatively more complex and some dopants like Mg, Ti, Nb, and Ru can decrease the barriers of the diffusion paths. The calculations also show the evidence of hole polaron formation in LiMnO2 and electron polaron formation in Li2MnO3 which should be the reason why these phases have low electronic conductivities. Based on these findings, possible ways to improve the electronic conductivity through the doping process are discussed.

 Please wait while we load your content...
Please wait while we load your content...