A facile approach of introducing DMS into LiODFB–PYR14TFSI electrolyte for lithium-ion batteries†
Abstract
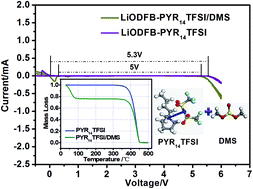
Ionic liquid-based electrolytes are widely used in lithium-ion batteries to obtain wide electrochemical windows and fewer safety concerns. In order to enhance the energy density and rate capability, lithium difluoro(oxalato)borate (LiODFB) and dimethyl sulfite (DMS) have been introduced into N-butyl-N-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide (PYR14TFSI)-based electrolytes as lithium salt and co-solvent, respectively. The binary LiODFB–PYR14TFSI/DMS (7 : 3, m/m) electrolyte has been prepared and investigated. FTIR analysis has demonstrated that stretching of the functional groups C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O–S–O plays a vital role in the solvation of the designed electrolyte, and elevate the ionic conductivity of the electrolyte by an order of magnitude. In comparison to when a LiTFSI–PYR14TFSI/DMS (7 : 3, m/m) electrolyte is used, Li/MCMB half-cells containing the novel electrolyte achieve excellent electrochemical performances, even superior to those of conventional organic electrolytes, maintaining a discharge capacity and coloumbic efficiency of 273.2 mA h g−1 and 99%, respectively, after 80 cycles. This tremendous improvement is ascribed to the joint reductive composition of DMS and LiODFB, resulting in the formation of a more robust solid-electrolyte interface (SEI) film at the MCMB electrode. Besides, the discharge capacity of Li/LiFePO4 half-cells with the LiODFB-based electrolyte could still reach up to 139 mA h g−1 at a rate of 1 C. All those characteristics make it a promising electrolyte material for safe and high-performance lithium-ion batteries.
O and O–S–O plays a vital role in the solvation of the designed electrolyte, and elevate the ionic conductivity of the electrolyte by an order of magnitude. In comparison to when a LiTFSI–PYR14TFSI/DMS (7 : 3, m/m) electrolyte is used, Li/MCMB half-cells containing the novel electrolyte achieve excellent electrochemical performances, even superior to those of conventional organic electrolytes, maintaining a discharge capacity and coloumbic efficiency of 273.2 mA h g−1 and 99%, respectively, after 80 cycles. This tremendous improvement is ascribed to the joint reductive composition of DMS and LiODFB, resulting in the formation of a more robust solid-electrolyte interface (SEI) film at the MCMB electrode. Besides, the discharge capacity of Li/LiFePO4 half-cells with the LiODFB-based electrolyte could still reach up to 139 mA h g−1 at a rate of 1 C. All those characteristics make it a promising electrolyte material for safe and high-performance lithium-ion batteries.

 Please wait while we load your content...
Please wait while we load your content...