Low-activated Li-ion mobility and metal to semiconductor transition in CdP2@Li phases†
Abstract
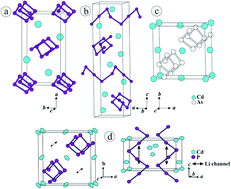
Solids with high ion mobility are of broad interest for energy storage applications. New systems featuring low-activated ion mobility are important to improve the performance in such systems. Herein we report on a model system dealing with such improved properties. Li0.2CdP2 was synthesized from the elements, lithium as structure stabilizer and CdI2 as reaction promoters in sealed silica ampoules at 823 K. It crystallizes tetragonal, in space group I4122 (α-CdAs2 structure type), with lattice parameters a = 7.6691(8) Å, c = 4.4467(4) Å and V = 261.53(4) Å3. After 24 h of storage in air lithium ions can be removed in a spontaneous delithiation reaction resulting in Li(OH)·H2O formation on the surface of the crystals. Formed α′-CdP2 adopts the α-CdAs2 structure type. Both compounds consist of isolated cadmium atoms and helical 1∞[P−]-chains generating empty channels suitable to accommodate Li ions. The heavy atom structure was determined by X-ray diffraction methods while a full model including lithium was derived from a combined solid state NMR and quantum chemical calculation approach. An low activation barrier range in the order of 0.1 to 0.2 eV was determined by NMR spectroscopy pointing towards an extraordinary high Li mobility in Li0.2CdP2. Of course a Cd-based solid will have certain disadvantages like toxicity and mass for storage applications but substitution of Cd by suitable lighter elements can solve this issue.

 Please wait while we load your content...
Please wait while we load your content...