Parametric investigation of room-temperature fluoride-ion batteries: assessment of electrolytes, Mg-based anodes, and BiF3-cathodes†
Abstract
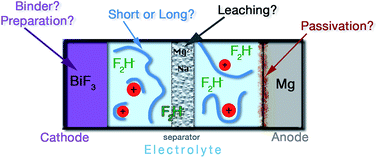
Little is known about room-temperature fluoride-ion batteries (RT FiBs), and no investigations that have varied their potential electrolytes, cathode preparations, or anode materials have been reported. In this publication, an overview of our recent investigations of these parameters for the purposes of improving the discharge capacities of RT FiBs is provided. The poly(ethylene glycol) (PEG)-based electrolytes in these systems function as ligands for fluoride ions. The impact of the increasing ligand length on battery capacity was investigated. Using Mg as an anode, different anode builds (e.g., foil or pressed pellets), as well as several composite anodes (e.g., Mg/MgF2) were tested. Furthermore the difference between cathodes prepared as hand-spread slurries or by spray-coating was investigated. Additionally the impact of using a water-soluble binder was examined. Finally, problems due to the leaching of alkaline (and/or alkaline earth) metal ions from the glass-fiber separators into the electrolyte were considered. In summary, it was demonstrated that FiBs will work using magnesium anodes, and that the capacities of such batteries are sensitive to every small change in their components.

 Please wait while we load your content...
Please wait while we load your content...