Facile preparation of rosin-based biochar coated bentonite for supporting α-Fe2O3 nanoparticles and its application for Cr(vi) adsorption†
Abstract
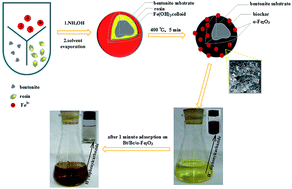
Pure nanometals have poor adsorption capacity due to their agglomeration in aqueous solution. In this study, biochar (Bc) prepared by the pyrolysis of rosin and coated on bentonite (Bt) was designed to support and disperse nanoscale α-Fe2O3 (Bt/Bc/α-Fe2O3). The generation of α-Fe2O3 nanoparticles and the pyrolysis of rosin on the surface of Bt were accomplished in a one-step heating route. Fourier transform infrared spectroscopy (FTIR) and X-ray diffraction (XRD) analysis confirmed the pyrolysis of rosin into biochar and the production of α-Fe2O3 in the composite. Besides, scanning electron microscopy (SEM) images showed good dispersion of α-Fe2O3 nanoparticles on the biochar surface. This adsorbent demonstrated fast Cr(VI) adsorption property with a Cr(VI) removal efficiency of 95% within one minute and high Cr(VI) adsorption capacity with a maximum Cr(VI) removal up to 81.7 mg g−1 based on the Langmuir model. The remarkable improvement of Cr(VI) adsorption on Bt/Bc/α-Fe2O3 was attributed to the good dispersion of α-Fe2O3 nanoparticles by the biochar network in comparison with other similar adsorbents. In addition, Bt/Bc/α-Fe2O3 maintained high Cr(VI) adsorption capacity under both acidic and basic conditions. This indicated that Bt/Bc/α-Fe2O3 can be used for fast Cr(VI) removal from wastewater or emergent Cr(VI) leakage due to its facile preparation and high efficiency in a wide range of pH.

 Please wait while we load your content...
Please wait while we load your content...