Analysis and characterization of iron pyrite nanocrystals and nanocrystalline thin films derived from bromide anion synthesis†
Abstract
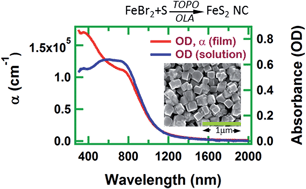
We use a solution-based hot injection method to synthesize stable, phase pure and highly crystalline cubic iron pyrite (FeS2) nanocrystals, with size varying from ∼70 to 150 nm. We use iron(II) bromide as an iron precursor, elemental sulfur as the sulfur source, trioctylphosphine oxide (TOPO) and 1,2-hexanediol as capping ligands, and oleylamine (OLA) as a non-coordinating solvent during the synthesis. We report on the influence of hydrazine treatment, and of thermal sintering, on the morphological, electronic, optical, and surface chemical properties of FeS2 films. Four point probe and Hall measurements indicate that these iron pyrite films are highly conductive. Although they are unsuitable as an effective photovoltaic light-absorbing layer, they offer clear potential as a conducting contact layer in photovoltaic and other optoelectronic devices.

 Please wait while we load your content...
Please wait while we load your content...