Porous perovskite calcium–manganese oxide microspheres as an efficient catalyst for rechargeable sodium–oxygen batteries†
Abstract
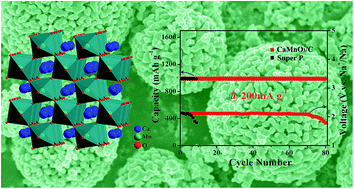
We report herein the preparation of porous CaMnO3 microspheres and their electrochemical catalytic performance as a cathode for rechargeable sodium–oxygen (Na–O2) batteries. In ether-based electrolytes, the CaMnO3/C cathode exhibits a high discharge capacity of 9560 mA h g−1 at a current density of 100 mA g−1, high rate capability (a capacity of 1940 mA h g−1 at 1000 mA g−1), and considerable cyclability up to 80 cycles. Two discharged species of NaO2 and Na2O2 are detected at the discharged state. The remarkable electrocatalytic activity of CaMnO3 both for the oxygen reduction reaction (ORR) and for the oxygen evolution reaction (OER) is attributed to the porous micro–nanostructures in stable ether-based electrolytes.

 Please wait while we load your content...
Please wait while we load your content...