A bottom-up synthesis of α-Fe2O3 nanoaggregates and their composites with graphene as high performance anodes in lithium-ion batteries†
Abstract
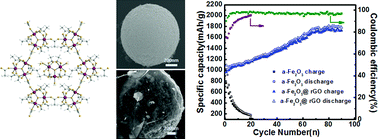
Nanoaggregates, novel nano/micron hierarchical structures with large surface area and significantly reduced grain boundary, are potentially useful electroactive materials. Herein, we report a facile, low-cost and scalable synthesis of α-Fe2O3 nanoaggregates by hydrothermally treating a single precursor of a star-shaped polymeric iron(III) acetate at 200 °C. The nanoaggregates possess a surface area of 47.84 m2 g−1 and a small crystalline size of 10 nm. The application of the nanoaggregates as the anode in a lithium ion battery was explored. Although the nanoaggregates cannot afford a large volume change during the lithium insertion/extraction process, the use of their core/shell composites with reduced graphene oxide (rGO) (α-Fe2O3@rGO) has led to a stable cycling performance over 90 cycles at a current density of 0.1 A g−1, a high specific capacity of 1787.27 mA h g−1 for the 90th cycle at 0.1 A g−1 and a good rate capability at various current densities. It is believed that the graphene shell helps to increase the conductivity of the composite electrodes, maintain the structural integrity of the nanoaggregates, enhance the mechanical stability of the electrode and confine a stabilized solid-electrolyte interphase (SEI) layer on the surface of the composite.

 Please wait while we load your content...
Please wait while we load your content...