Optical, electronic, and photoelectrochemical properties of the p-type Cu3−xVO4 semiconductor†
Abstract
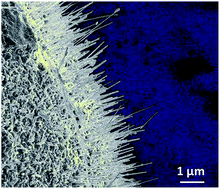
Investigations into new p-type metal oxides with small bandgap sizes, i.e., Eg ∼0.9 eV to ∼1.5 eV, are currently needed to enable the preparation of tandem cells with high solar-to-hydrogen conversion efficiencies. The p-type Cu(I)-vanadate, Cu3VO4 (space group I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2m (no. 121), Z = 2, a = 4.581(4) Å, c = 8.998(2) Å), was synthesized in high purity using solid-state methods and investigated for its small optical bandgap size (Eg ∼ 1.2 eV) and photoelectrochemical properties in the form of polycrystalline films. Powder X-ray diffraction and electron microscopy data show that, beginning at 300 °C in air, a Cu-deficient composition is formed according to: Cu3VO4(s) + (x/2) O2(g) → Cu3−xVO4(s) + x CuO(s). At 350 °C the compound decomposes at the surfaces into the Cu(II)-containing oxides CuO and Cu3V2O8 (3 : 1 molar ratio), the latter protruding as rods (i.e., ∼15–25 nm in width by ∼1–5 μm in length) from the particles' surfaces. Polycrystalline films of p-type Cu3VO4 were prepared under these conditions (i.e., heated in air at 300 °C or 350 °C) and found to yield significant cathodic photocurrents under solar-simulated, visible-light irradiation (λ > 420 nm; AM 1.5 G filter, irradiant power density of ∼100 mW cm−2). Their photocurrents increased with the heating temperature of the film and with the applied bias, e.g., ∼0.1 mA cm−2 at zero applied bias to ∼0.25 mA cm−2 at −0.2 applied bias (pH = 5.8). The photocurrent of a non-heated film was negligible compared to the films heated in air and exhibited a larger dark current. Further, when CuO nanoparticles were formed directly onto the Cu3VO4 films from aqueous Cu(NO3)2 solutions (i.e., 0.1 M and 0.25 M), cathodic photocurrents of ∼0.2 mA cm−2 are similarly found. Mott–Schottky measurements determined the energetic potential of the Cu3VO4 conduction band to be at ∼−0.63 V versus RHE at pH = 5.8, with an acceptor concentration of ∼1.29 × 1017 cm−3. Thus, a type-II band offset is predicted to occur between the p-type Cu3VO4 film and the p-type CuO surface nanoparticles, and elucidating the critical role of the CuO surface nanoparticles in forming a charge rectification barrier and enhancing the charge separation at the surfaces. Electronic structure calculations show that the conduction band states of Cu3VO4 are delocalized within the ab-plane of the structure and exhibit an ∼2 eV band dispersion centered around the gamma k-point. The bandgap size, conduction band dispersion, and band energies of Cu3VO4 are thus found to be promising for further investigations into tandem n-/p-type photoelectrochemical cells for solar energy conversion applications.
2m (no. 121), Z = 2, a = 4.581(4) Å, c = 8.998(2) Å), was synthesized in high purity using solid-state methods and investigated for its small optical bandgap size (Eg ∼ 1.2 eV) and photoelectrochemical properties in the form of polycrystalline films. Powder X-ray diffraction and electron microscopy data show that, beginning at 300 °C in air, a Cu-deficient composition is formed according to: Cu3VO4(s) + (x/2) O2(g) → Cu3−xVO4(s) + x CuO(s). At 350 °C the compound decomposes at the surfaces into the Cu(II)-containing oxides CuO and Cu3V2O8 (3 : 1 molar ratio), the latter protruding as rods (i.e., ∼15–25 nm in width by ∼1–5 μm in length) from the particles' surfaces. Polycrystalline films of p-type Cu3VO4 were prepared under these conditions (i.e., heated in air at 300 °C or 350 °C) and found to yield significant cathodic photocurrents under solar-simulated, visible-light irradiation (λ > 420 nm; AM 1.5 G filter, irradiant power density of ∼100 mW cm−2). Their photocurrents increased with the heating temperature of the film and with the applied bias, e.g., ∼0.1 mA cm−2 at zero applied bias to ∼0.25 mA cm−2 at −0.2 applied bias (pH = 5.8). The photocurrent of a non-heated film was negligible compared to the films heated in air and exhibited a larger dark current. Further, when CuO nanoparticles were formed directly onto the Cu3VO4 films from aqueous Cu(NO3)2 solutions (i.e., 0.1 M and 0.25 M), cathodic photocurrents of ∼0.2 mA cm−2 are similarly found. Mott–Schottky measurements determined the energetic potential of the Cu3VO4 conduction band to be at ∼−0.63 V versus RHE at pH = 5.8, with an acceptor concentration of ∼1.29 × 1017 cm−3. Thus, a type-II band offset is predicted to occur between the p-type Cu3VO4 film and the p-type CuO surface nanoparticles, and elucidating the critical role of the CuO surface nanoparticles in forming a charge rectification barrier and enhancing the charge separation at the surfaces. Electronic structure calculations show that the conduction band states of Cu3VO4 are delocalized within the ab-plane of the structure and exhibit an ∼2 eV band dispersion centered around the gamma k-point. The bandgap size, conduction band dispersion, and band energies of Cu3VO4 are thus found to be promising for further investigations into tandem n-/p-type photoelectrochemical cells for solar energy conversion applications.

 Please wait while we load your content...
Please wait while we load your content...