Dependence of solvent quality on the composition of copolymers: experiment and theory for solutions of P(MMA-ran-t-BMA) in toluene and in chloroform
Abstract
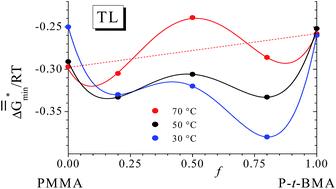
The interaction of toluene with P(MMA-ran-t-BMA) and with the corresponding homopolymers was determined via vapor pressure measurements at 30, 50 and 70 °C. A unified thermodynamic approach served for the modeling of the results. It is capable of describing the behavior of the different solutions by means of two adjustable parameters, one representing the effective number of solvent segments and the other accounting for the interactions between the components. The solvent quality of toluene passes a maximum, a minimum and another maximum upon an increase of the t-BMA content of the copolymer at all temperatures. A similar behavior is discernable from vapor pressure data of chloroform published for the same copolymers. The heats of mixing for toluene depend strongly on temperature; at 50 °C they are all endothermal with the exception of PMMA, for which the value obtained from vapor pressures at 30 °C agrees very well with published caloric data.

 Please wait while we load your content...
Please wait while we load your content...