Aminofluorination: transition-metal-free N–F bond insertion into diazocarbonyl compounds†
Abstract
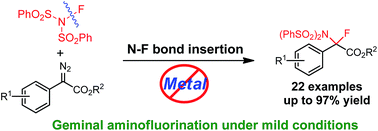
Gem-aminofluorination of diazocarbonyl compounds has been achieved for the first time. This reaction proceeds under mild conditions and does not require any transition-metal promoter or catalyst. Treatment of diazoesters with N-fluorobenzenesulfonimide (NFSI), which serves as both a fluorine and nitrogen source, results in the facile construction of C–N and C–F bonds, providing aminofluorination products in moderate to excellent yields. Kinetic studies and DFT calculations have provided valuable insight into the potential mechanism for this novel N–F bond insertion.
- This article is part of the themed collection: Top 50 Articles of 2016: Organic Chemistry

 Please wait while we load your content...
Please wait while we load your content...