Spatial separation of oxidation and reduction co-catalysts for efficient charge separation: Pt@TiO2@MnOx hollow spheres for photocatalytic reactions†
Abstract
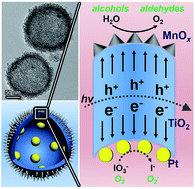
Efficient charge separation is a critical factor for solar energy conversion by heterogeneous photocatalysts. This paper describes the complete spatial separation of oxidation and reduction cocatalysts to enhance the efficacy of charge separation and surface reaction. Specifically, we design Pt@TiO2@MnOx hollow spheres (PTM-HSs) with Pt and MnOx loaded onto the inner and outer surface of TiO2 shells, respectively. Pt favours electron trapping, while MnOx tends to collect holes. Upon generation from TiO2, electrons and holes flow inward and outward of the spherical photocatalyst, accumulating on the corresponding cocatalysts, and then take part in redox reactions. Combined with other advantages, such as the large surface area and appropriate pore size, the PTM-HSs exhibit high efficiency for the photocatalytic oxidation of water and benzyl alcohol. The mechanism of the oxidation process of benzyl alcohol over the photocatalyst is also presented.
- This article is part of the themed collections: Global Energy Challenges: Solar Energy and Global Energy Challenges: Energy Applications

 Please wait while we load your content...
Please wait while we load your content...