A diversity-oriented synthesis of bioactive benzanilides via a regioselective C(sp2)–H hydroxylation strategy†
Abstract
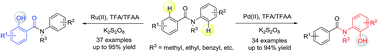
A diversity-oriented synthesis of bioactive benzanilides via C(sp2)–H hydroxylation has been studied. Different regioselectivity was observed with Ru(II) and Pd(II) catalysts. The reaction demonstrates excellent regioselectivity, good tolerance of functional groups, and high yields. A wide range of ortho-hydroxylated-benzanilides can be readily synthesized with excellent regioselectivity via this new synthetic strategy. Computational investigations revealed that the regioselectivity was controlled mainly by both steric and electronic factors. Steric effects determine the regioselective outcomes in the Ru-catalyzed reaction, while electronic effects are dominant in the Pd-catalyzed reaction.


 Please wait while we load your content...
Please wait while we load your content...