A dual-responsive nanocapsule via disulfide-induced self-assembly for therapeutic agent delivery†
Abstract
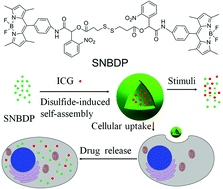
One-step synthesis of fluorescent molecules (SNBDP) containing one disulfide bond and two o-nitrobenzyl groups was demonstrated via multi-component Passerini reaction. This hydrophobic SNBDP could self-assemble into nanocapsules (SNBDP NCs) in aqueous solution via disulfide-induced assembly. The obtained nanocapsules were stable in aqueous solution for several weeks and exhibited enhanced fluorescence when nanocapsules were destroyed due to disaggregation-induced emission. The nanocapsules not only were reduction-sensitive and light-responsive, but also could be endocytosed by HeLa cells for cellular imaging. The enhanced fluorescence in the glutathione (GSH) pretreated HeLa cells showed that the compound was reduction-sensitive in living cells. In vitro WST-8 assays showed the nanocapsules were biocompatible and could further be used as drug delivery carriers. Indocyanine green (ICG), a clinically approved NIR dye, was loaded into the nanocapsules (ICG@SNBDP NCs). ICG@SNBDP NCs showed enhanced photothermal efficacy compared with same concentration of free ICG under 808-nm laser irradiation. Consequently, ICG@SNBDP NCs upon NIR irradiation can effectively kill cancer cells through local hyperthermia. These results highlight the potential of disulfide-induced nanocapsules as smart nanoparticles for cellular imaging and therapeutic agent delivery.

 Please wait while we load your content...
Please wait while we load your content...