Direct α-heteroarylation of amides (α to nitrogen) and ethers through a benzaldehyde-mediated photoredox reaction†
Abstract
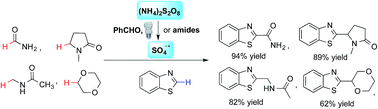
A photoredox reaction for cross-dehydrogenative coupling (CDC) was developed to Cα-arylate amides (α to nitrogen) and ethers using a variety of five- and six-membered electron-deficient heteroarenes. A unique decomposition mechanism of ammonium persulfate enhanced by photoexcited benzaldehydes was revealed. This benzaldehyde-mediated photoredox reaction proceeded smoothly with household 23 W CFL bulbs as the energy source under metal-free conditions, allowing the construction of new Csp2–Csp2 and Csp3–Csp2 bonds and access to important pharmacophores of broad utility using commercially available reagents.


 Please wait while we load your content...
Please wait while we load your content...