Chiral phosphine-catalyzed tunable cycloaddition reactions of allenoates with benzofuranone-derived olefins for a highly regio-, diastereo- and enantioselective synthesis of spiro-benzofuranones†
Abstract
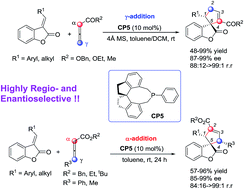
The first regioselective catalytic asymmetric [3 + 2] cycloaddition of benzofuranone-derived olefins with allenoates and substituted allenoates has been developed in the presence of (R)-SITCP, affording different functionalized 3-spirocyclopentene benzofuran-2-ones in good yields with high enantioselectivities under mild conditions. The substrate scope has also been examined. The regioselective outcomes for this phosphine-catalyzed [3 + 2] cycloaddition reaction can be rationalized using DFT calculations.

 Please wait while we load your content...
Please wait while we load your content...