Metal-only Lewis pairs between group 10 metals and Tl(i) or Ag(i): insights into the electronic consequences of Z-type ligand binding†
Abstract
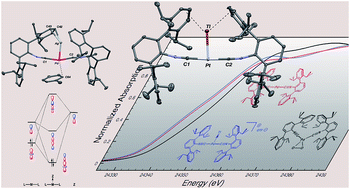
Complexes bearing electron rich transition metal centers, especially those displaying coordinative unsaturation, are well-suited to form reverse-dative σ-interactions with Lewis acids. Herein we demonstrate the generality of zerovalent, group 10 m-terphenyl isocyanide complexes to form reverse-dative σ-interactions to Tl(I) and Ag(I) centers. Structural and spectroscopic investigations of these metal-only Lewis pairs (MOLPs) has allowed insight into the electronic consequences of Lewis-acid ligation within the primary coordination sphere of a transition metal center. Treatment of the bis-isocyanide complex, Pt(CNArDipp2)2 (ArDipp2 = 2,6-(2,6-(i-Pr)2C6H3)2C6H3) with TlOTf (OTf = [O3SCF3]−) yields the Pt/Tl MOLP [TlPt(CNArDipp2)2]OTf (1). 1H NMR and IR spectroscopic studies on 1, and its Pd congener [TlPd(CNArDipp2)2]OTf (2), demonstrate that the M → Tl interaction is labile in solution. However, treatment of complexes 1 and 2 with Na[BArF4] (ArF = 3,5-(CF3)2C6H3) produces [TlPt(CNArDipp2)2]BArF4 (3) and [TlPd(CNArDipp2)2]BArF4 (4), in which Tl(I) binding is shown to be static by IR spectroscopy and, in the case of 3, 195Pt NMR spectroscopy as well. This result provides strong evidence that the M → Tl linkages can be attributed primarily to σ-donation from the group 10 metal to Tl, as loss of ionic stabilization of Tl by the triflate anion is compensated for by increasing the degree of M → Tl σ-donation. In addition, X-ray Absorption Near-Edge Spectroscopy (XANES) on the Pd/Tl and Ni/Tl MOLPs, [TlPd(CNArDipp2)2]OTf (2) and [TlNi(CNArMes2)3]OTf, respectively, is used to illustrate that the formation of a reverse-dative σ-interaction with Tl(I) does not alter the spectroscopic oxidation state of the group 10 metal. Also reported is the ability of M(CNArDipp2)2 (M = Pt, Pd) to form MOLPs with Ag(I), yielding the complexes [AgM(CNArDipp2)2]OTf (5, M = Pt; 6, M = Pd). As was determined for the Tl-containing MOLPs 1–4, it is shown that the spectroscopic oxidation states of the group 10 metal in 5 and 6 are essentially unchanged compared to the zerovalent precursors M(CNArDipp2)2. However, in the case of 5 and 6, the formation of a dative M → Ag σ-bonding interaction facilitates the binding of Lewis bases to the group 10 metal trans to Ag, illustrating the potential of acceptor fragments to open up new coordination sites on transition metal complexes without formal, two-electron oxidation.

 Please wait while we load your content...
Please wait while we load your content...