A straightforward method for automated Fmoc-based synthesis of bio-inspired peptide crypto-thioesters†
Abstract
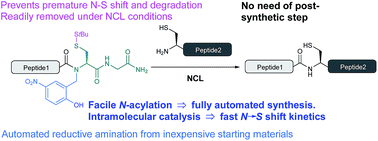
Despite recent advances, the direct Fmoc-based solid phase synthesis of peptide α-thioesters for the convergent synthesis of proteins via native chemical ligation (NCL) remains a challenge in the field. We herein report a simple and general methodology, enabling access to peptide thioester surrogates. A novel C-terminal N-(2-hydroxybenzyl)cysteine thioesterification device based on an amide-to-thioester rearrangement was developed, and the resulting peptide crypto-thioesters can be directly used in NCL reactions with fast N → S shift kinetics at neutral pH. These fast kinetics arise from our bio-inspired design, via intein-like intramolecular catalysis. Due to a well-positioned phenol moiety, an impressive >50 fold increase in the kinetic rate is observed compared to an O-methylated derivative. Importantly, the synthesis of this new device can be fully automated using inexpensive commercially available materials and does not require any post-synthetic steps prior to NCL. We successfully applied this new method to the synthesis of two long naturally-occurring cysteine-rich peptide sequences.

 Please wait while we load your content...
Please wait while we load your content...