Control of cerium oxidation state through metal complex secondary structures†
Abstract
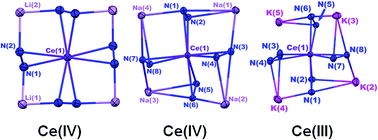
A series of alkali metal cerium diphenylhydrazido complexes, Mx(py)y[Ce(PhNNPh)4], M = Li, Na, and K, x = 4 (Li and Na) or 5 (K), and y = 4 (Li), 8 (Na), or 7 (K), were synthesized to probe how a secondary coordination sphere would modulate electronic structures at a cerium cation. The resulting electronic structures of the heterobimetallic cerium diphenylhydrazido complexes were found to be strongly dependent on the identity of the alkali metal cations. When M = Li+ or Na+, the cerium(III) starting material was oxidized with concomitant reduction of 1,2-diphenylhydrazine to aniline. Reduction of 1,2-diphenylhydrazine was not observed when M = K+, and the complex remained in the cerium(III) oxidation state. Oxidation of the cerium(III) diphenylhydrazido complex to the Ce(IV) diphenylhydrazido one was achieved through a simple cation exchange reaction of the alkali metals. UV-Vis spectroscopy, FTIR spectroscopy, electrochemistry, magnetic susceptibility, and DFT studies were used to probe the oxidation state and the electronic changes that occurred at the metal centre.

 Please wait while we load your content...
Please wait while we load your content...