Bipyridine complexes of E3+ (E = P, As, Sb, Bi): strong Lewis acids, sources of E(OTf)3 and synthons for EI and EV cations†
Abstract
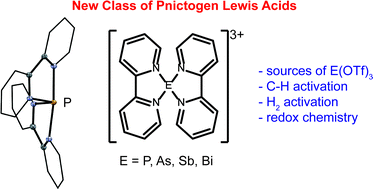
Triflate salts of trications [(bipy)2E]3+ ([6E][OTf]3) and [(tbbipy)2E]3+ ([6′E][OTf]3) (bipy = 2,2′-bipyridine, tbbipy = 4,4′-di-tbutyl-2,2′-bipyridine; E = P, As, Sb, Bi) have been synthesized and comprehensively characterized. The unique molecular and electronic structures of this new class of complexes involving pnictogen Lewis acids has been assessed in the solid, solution and gas phases to reveal systematic variations in metric parameters, ligand lability and charge concentration. While the Lewis acidity of E3+ has the trend E = Bi < Sb < As < P as determined by gas-phase calculations and 1H NMR spectroscopy, the Lewis acidity of [6E]3+ has the trend E = P < As < Sb < Bi according to gas-phase calculations. Derivatives of [6′E][OTf]3 (E = P, As) are latent sources of E(OTf)3 as demonstrated by their reactions with dmap, which give the corresponding derivatives of [(dmap)3E][OTf]3. The highly oxidizing nature of P(OTf)3 and As(OTf)3 is evidenced in reactions of [6′E][OTf]3 (E = P, As) with phosphines, which give EI-containing monocations [(R3P)2E]1+ and oxidatively coupled dications [R3PPR3]2+, illustrating new P–P and P–As bond forming strategies. Cations [6′E]3+ (E = P, As) are C–H bond activating agents that dehydrogenate 1,4-cyclohexadiene, with higher activity observed for E = P. Combinations of [6′E]3+ and tBu3P activate H2 and D2 under mild conditions, evidencing frustrated Lewis pair activity. Oxidation of [6′P][OTf]3 with SO2Cl2 gives [(tbbipy)2PCl2][OTf]3, containing a PV-trication, but there is no evidence of the analogous reaction with [6′As][OTf]3. The observations highlight new directions in the chemistry of highly charged cations and reveal a rich reactivity for p-block triflates E(OTf)3, which can be accessed through derivatives of [6E][OTf]3 and [6′E][OTf]3.

 Please wait while we load your content...
Please wait while we load your content...