Construction of highly functionalized carbazoles via condensation of an enolate to a nitro group†
Abstract
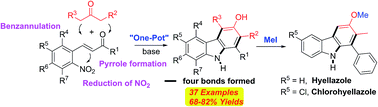
This paper describes a novel synthesis of highly functionalized and diverse carbazoles via transition-metal-free and mild base-promoted condensations of readily available 2-nitrocinnamaldehyde or 2-nitrochalcones with various β-ketoesters or 1,3-diaryl-2-propanones. The method selectively forms four bonds by the intramolecular conjugate addition of an enolate to the enal or chalcone bearing an o-nitro group. This group then undergoes in situ N–O bond cleavage under non-reductive conditions in a one-pot procedure. This protocol allows for the introduction of various functional groups at all positions of the newly formed aromatic ring of the carbazole moiety. The utility of this methodology is further illustrated by the concise synthesis of naturally occurring hyellazole and chlorohyellazole.


 Please wait while we load your content...
Please wait while we load your content...