Copper-catalyzed condensation of imines and α-diazo-β-dicarbonyl compounds: modular and regiocontrolled synthesis of multisubstituted pyrroles†
Abstract
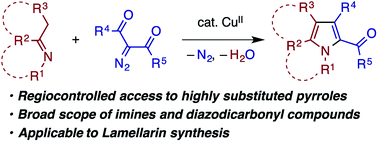
In the presence of a copper(II) catalyst, enolizable imines bearing various N-substituents and α-diazo-β-ketoesters undergo denitrogenative and dehydrative condensation to afford highly substituted pyrroles in moderate to good yields with exclusive regioselectivity. The reaction likely involves nucleophilic addition of the imine nitrogen to a copper carbenoid, tautomerization of the resulting azomethine ylide to an α-enaminoketone, and a subsequent enamine–ketone cyclocondensation. With Yb(OTf)3 as a unique cocatalyst, α-diazo-β-diketones also participate in the same condensation reaction. The present reaction is applicable to acyclic, exocyclic, and endocyclic imines with tolerance of a broad range of functional groups and heterocyclic moieties, thus opening a new convenient route for the synthesis of the lamellarin family of natural products.

 Please wait while we load your content...
Please wait while we load your content...