Copper-catalyzed direct transformation of simple alkynes to alkenyl nitriles via aerobic oxidative N-incorporation†
Abstract
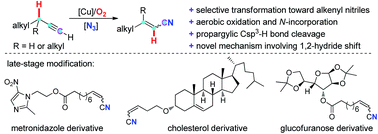
A novel direct transformation of aliphatic terminal alkynes to alkenyl nitriles through the incorporation of a nitrogen atom into the simple hydrocarbons has been reported. The usage of inexpensive copper catalyst, O2 as the sole oxidant, broad substrate scope as well as feasibility for “late-stage modification” make this protocol very promising. Mechanistic studies including DFT calculation demonstrate a novel 1,2-hydride shift process for this novel nitrogenation reaction.

 Please wait while we load your content...
Please wait while we load your content...