Rational synthesis of normal, abnormal and anionic NHC–gallium alkyl complexes: structural, stability and isomerization insights†‡
Abstract
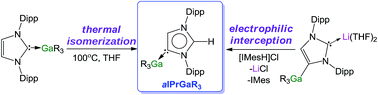
Advancing the rational design of main-group N-heterocyclic carbene complexes, this study reports the synthesis, X-ray crystallographic and NMR spectroscopic characterisation of a novel series of Ga complexes containing neutral or anionic NHC ligands using the unsaturated carbene IPr (IPr = 1,3-bis-(2,6-di-isopropylphenyl)imidazol-2-ylidene). Starting from normal adduct GaR3·IPr (1) (R = CH2SiMe3), the addition of polar LiR led to the formation of NHC-stabilised gallate species IPr·LiGaR4 (2), resulting from co-complexation of the single-metal species. Contrastingly, reversing the order of addition of these organometallic reagents, by treating unsaturated free IPr, first with LiR followed by GaR3, furnished novel heteroleptic gallate (THF)2Li[:C{[N(2,6-iPr2C6H3)]2CHCGa(CH2SiMe3)3}] (3), which contains an anionic NHC ligand acting as an unsymmetrical bridge between the two metals, coordinating through its abnormal C4 position to Ga and through its normal C2 position to Li. Electrophilic interception studies of 3 using methyl triflate (MeOTf), methanol and imidazolium salt (IMes·HCl) led to the isolation and structural elucidation of the two novel neutral abnormal NHC (aNHC) complexes [CH3C{[N(2,6-iPr2C6H3)]2CHCGa(CH2SiMe3)3}] (4) and aIPr·GaR3 (5) (aIPr = HC{[N(2,6-iPr2C6H3)]2CHC}). These studies disclose the preference of the anionic IPr ligand present in 3 to react with electrophiles via its C2 position, leaving its Ga–C4 bond intact. Abnormal complex 5 can also be accessed by a thermally induced rearrangement of its normal isomer 1. Combining NMR spectroscopic and kinetic studies with DFT calculations, new light has been shed on this intriguing transformation, which suggests that it occurs via a dissociative mechanism, highlighting the importance of the donor ability of the solvent used in these thermal isomerizations as well as the steric bulk of the substituents on the NHC and the Ga reagent. These findings intimate that relief of the steric hindrance around Ga by forming an abnormal complex is a key driving force behind these rearrangements.
- This article is part of the themed collection: Celebrating the 2017 RSC Prize and Award Winners

 Please wait while we load your content...
Please wait while we load your content...