Ligand-enabled Ir-catalyzed intermolecular diastereoselective and enantioselective allylic alkylation of 3-substituted indoles†
Abstract
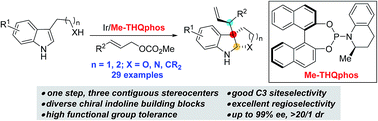
An Ir-catalyzed asymmetric allylic alkylation of 3-substituted indoles is reported. The reaction provides indoline products containing multiple contiguous stereocenters with high site-, regio-, diastereo- and enantioselectivities in one step from a wide range of readily available starting materials. The key to this method is the high level of diastereocontrol enabled by an iridium catalyst derived from a N-aryl phosphoramidite ligand (Me-THQphos, 1c).

 Please wait while we load your content...
Please wait while we load your content...