A push–pull unsymmetrical subphthalocyanine dimer†
Abstract
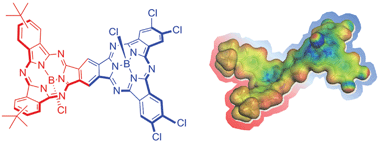
Unsymmetrical subphthalocyanine fused dimers have been prepared from appropriate ortho-dinitrile SubPc precursors. In particular, either electron-donating or electron-accepting substituents have been introduced on each SubPc constituent unit, resulting in unprecedented push–pull π-extended curved aromatic macrocycles. From fluorescence experiments in solvents of different polarity we conclude a dual fluorescence, namely a delocalized singlet excited state (1.73 eV) and a polarized charge transfer state (<1.7 eV). Pump probe experiments corroborate the dual nature of the fluorescence. On one hand, the delocalized singlet excited state gives rise to a several nanosecond lasting intersystem crossing yielding the corresponding triplet excited state. On the other hand, the polarized charge transfer state deactivates within a few picosesonds. Visualization of the charge transfer state was accomplished by means of molecular modeling with a slight polarization of the HOMO towards the electron donor and of the LUMO towards the electron acceptor.


 Please wait while we load your content...
Please wait while we load your content...