Overcoming aggregation in indium salen catalysts for isoselective lactide polymerization†
Abstract
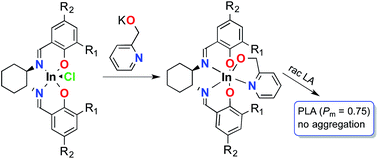
A methodology for controlling aggregation in highly active and isoselective indium catalysts for the ring opening polymerization of racemic lactide is reported. A series of racemic and enantiopure dinuclear indium ethoxide complexes bearing salen ligands [(ONNOR)InOEt]2 (R = Br, Me, admantyl, cumyl, t-Bu) were synthesized and fully characterized. Mononuclear analogues (ONNOR)InOCH2Pyr (R = Br, t-Bu, SiPh3) were synthesized by controlling aggregation with the use of chelating 2-pyridinemethoxide functionality. The nuclearity of metal complexes was confirmed using PGSE NMR spectroscopy. Detailed kinetic studies show a clear initiation period for these dinuclear catalysts, which is lacking in their mononuclear analogues. The polymerization behavior of analogous dinuclear and mononuclear compounds is identical and consistent with a mononuclear propagating species. The isotacticity of the resulting polymers was investigated using direct integration and peak deconvolution methodologies and the two were compared.

 Please wait while we load your content...
Please wait while we load your content...