Systematic re-evaluation of the bis(2-hydroxyethyl)disulfide (HEDS) assay reveals an alternative mechanism and activity of glutaredoxins†
Abstract
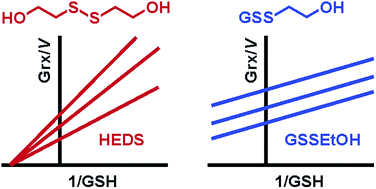
The reduction of bis(2-hydroxyethyl)disulfide (HEDS) by reduced glutathione (GSH) is the most commonly used assay to analyze the presence and properties of enzymatically active glutaredoxins (Grx), a family of central redox proteins in eukaryotes and glutathione-utilizing prokaryotes. Enzymatically active Grx usually prefer glutathionylated disulfide substrates. These are converted via a ping-pong mechanism. Sequential kinetic patterns for the HEDS assay have therefore been puzzling since 1991. Here we established a novel assay and used the model enzyme ScGrx7 from yeast and PfGrx from Plasmodium falciparum to test several possible causes for the sequential kinetics such as pre-enzymatic GSH depletion, simultaneous binding of a glutathionylated substrate and GSH, as well as substrate or product inhibition. Furthermore, we analyzed the non-enzymatic reaction between HEDS and GSH by HPLC and mass spectrometry suggesting that such a reaction is too slow to explain high Grx activities in the assay. The most plausible interpretation of our results is a direct Grx-catalyzed reduction of HEDS. Physiological implications of this alternative mechanism and of the Grx-catalyzed reduction of non-glutathione disulfide substrates are discussed.

 Please wait while we load your content...
Please wait while we load your content...