Sugar silanes: versatile reagents for stereocontrolled glycosylation via intramolecular aglycone delivery†
Abstract
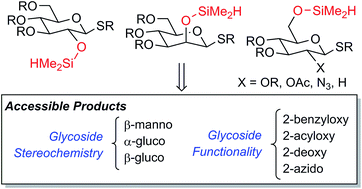
A new method for the intramolecular glycosylation of alcohols is described. Utilizing carbohydrate-derived silanes, the catalytic dehydrogenative silylation of alcohols is followed by intramolecular glycosylation. Appropriate combinations of silane position and protecting groups allow highly selective access to β-manno, α-gluco, or β-gluco stereochemical relationships as well as 2-azido-2-deoxy-β-gluco- and 2-deoxy-β-glucosides. Intramolecular aglycone delivery from the C-2 or C-6 position provides 1,2-cis or 1,2-trans glycosides, respectively. Multifunctional acceptor substrates such as hydroxyketones and diols are tolerated and are glycosylated in a site-selective manner.

 Please wait while we load your content...
Please wait while we load your content...