Rapid access to phospholipid analogs using thiol-yne chemistry†
Abstract
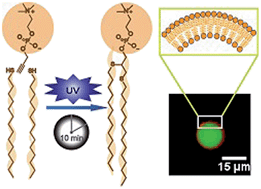
Phospholipids and glycolipids constitute an essential part of biological membranes, and are of tremendous fundamental and practical interest. Unfortunately, the preparation of functional phospholipids, or synthetic analogs, is often synthetically challenging. Here we utilize thiol-yne click chemistry methodology to gain access to phospho- and glycolipid analogs. Alkynyl hydrophilic head groups readily photoreact with numerous thiol modified lipid tails to yield the appropriate dithioether phospho- or glycolipids. The resulting structures closely resemble the structure and function of native diacylglycerolipids. Dithioether phosphatidylcholines (PCs) are suitable for forming giant unilamellar vesicles (GUV), which can be used as vessels for cell-free expression systems. The unnatural thioether linkages render the lipids resistant to phospholipase A2 hydrolysis. We utilize the improved stability of these lipids to control the shrinkage of GUVs composed of a mixture of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) and dioleyl-dithioether PC, concentrating encapsulated nanoparticles. We imagine that these readily accessible lipids could find a number of applications as natural lipid substitutes.

 Please wait while we load your content...
Please wait while we load your content...