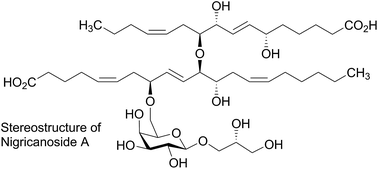
Structure elucidation of nigricanoside A through enantioselective total synthesis†
Abstract
Nigricanoside A was isolated from green alga, and its dimethyl ester was found to display potent cytotoxicity. Its scarcity prevented a full structure elucidation, leaving total synthesis as the only means to determine its relative and absolute stereochemistry and to explore its biological activity. Here we assign the stereochemistry of the natural product through enantioselective total synthesis and provide initial studies of its cytotoxicity.

 Please wait while we load your content...
Please wait while we load your content...