Enantioselective installation of adjacent tertiary benzylic stereocentres using lithiation–borylation–protodeboronation methodology. Application to the synthesis of bifluranol and fluorohexestrol†
Abstract
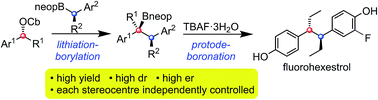
1,2-Diaryl ethanes bearing 1,2-stereogenic centres show interesting biological activity but their stereocontrolled synthesis has not been reported forcing a reliance of methods involving diastereomer and enantiomer separation. We have found that this class of molecules can be prepared with very high stereocontrol using lithiation–borylation methodology. The reaction of an enantioenriched benzylic lithiated carbamate with an enantioenriched benzylic secondary pinacol boronic ester gave a tertiary boronic ester with complete diastereo- and enantiocontrol. It was essential to use MgBr2/MeOH after formation of the boronate complex, both to promote the 1,2-migration and to trap any lithiated carbamate/benzylic anion that formed from fragmentation of the ate complex, anions that would otherwise racemise and re-form the boronate complex eroding both er and dr of the product. When the benzylic lithiated carbamate and benzylic secondary pinacol boronic ester were too hindered, boronate complex did not even form. In these cases, it was found that the use of the less hindered neopentyl boronic esters enabled successful homologation to take place even for the most hindered reaction partners, with high stereocontrol and without the need for additives. Protodeboronation of the product boronic esters with TBAF gave the target 1,2-diaryl ethanes bearing 1,2-stereogenic centres. The methodology was applied to the stereocontrolled synthesis of bifluranol and fluorohexestrol in just 7 and 5 steps, respectively.

 Please wait while we load your content...
Please wait while we load your content...