Stereocontrolled protein surface recognition using chiral oligoamide proteomimetic foldamers†
Abstract
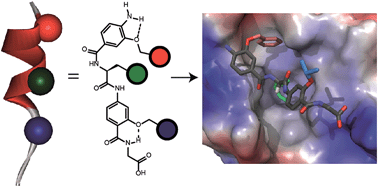
The development of foldamers capable of selective molecular recognition of solvent exposed protein surfaces represents an outstanding challenge in supramolecular chemical biology. Here we introduce an oligoamide foldamer with well-defined conformation that bears all the hallmarks of an information rich oligomer. Specifically, the foldamer recognizes its target protein hDM2 leading to inhibition of its protein–protein interaction with p53 in a manner that depends upon the composition, spatial projection and stereochemistry of functional groups appended to the scaffold. Most significantly, selective inhibition of p53/hDM2 can be achieved against four other targets and the selectivity for p53/hDM2 inhibition versus Mcl-1/NOXA-B inhibition is critically dependent upon the stereochemistry of the helix mimetic.
- This article is part of the themed collections: Global challenges: Health & Food and Celebrating the 2016 RSC Prize and Award Winners

 Please wait while we load your content...
Please wait while we load your content...