Thymine functionalised porphyrins, synthesis and heteromolecular surface-based self-assembly†
Abstract
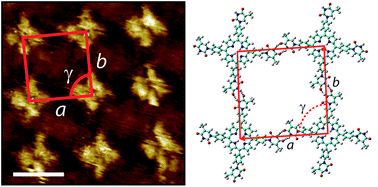
The synthesis and surface-based self-assembly of thymine-functionalised porphyrins is described. Reaction of 1-formylphenyl-3-benzoyl-thymine with suitable pyrollic species leads to the formation of tetra-(phenylthymine)porphyrin (tetra-TP) or mono-thymine-tri-(3,5-di-tert-butylphenyl)porphyrin (mono-TP). Single crystal X-ray diffraction studies demonstrate the self-association of mono-TP in the solid state through thymine⋯thymine hydrogen-bonding interactions but in solution this interaction (Kd = 6.1 ± 3.0 M−1) is relatively weak in comparison to the heteromolecular interaction between mono-TP and 9-propyladenine (K = 91.8 ± 20.5 M−1). STM studies of the tetratopic hydrogen-bonding tecton, tetra-TP, deposited on an HOPG substrate reveal the formation of an almost perfectly square self-assembled lattice through thymine⋯thymine hydrogen-bonding. Co-deposition of tetra-TP with 9-propyladenine leads to the adoption of preferable thymine⋯adenine interactions leading to the formation of a heteromolecular tetra-TP⋯9-propyladenine hydrogen bonded array including both Watson–Crick thymine⋯adenine interactions and adenine⋯adenine hydrogen-bonding. The studies demonstrate a pathway for the self-assembly of tetratopic hydrogen-bonding tectons and the use of preferential heteromolecular thymine⋯adenine interactions for the disruption of the homomolecular tetra-TP array. Studies of the self-assembly of tetra-TP and 9-propyladenine demonstrate a strong dependence on overall concentration and molar ratio of components indicating the importance of kinetic effects in surface self-assembly processes.
- This article is part of the themed collection: Celebrating the 2016 RSC Prize and Award Winners

 Please wait while we load your content...
Please wait while we load your content...