Structural characterization of holo- and apo-myoglobin in the gas phase by ultraviolet photodissociation mass spectrometry†
Abstract
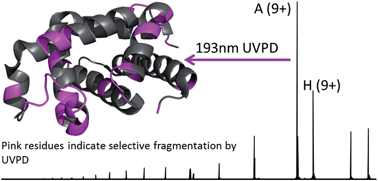
Ultraviolet photodissociation (UVPD) mass spectrometry is employed to investigate the structure of holo-myoglobin as well as its apo form transferred to the gas phase by native electrospray. UVPD provided insight into the stability of native structural elements of holo-myoglobin. The fragmentation yields from UVPD showed the greatest overall correlation with B-factors generated from the crystal structure of apo-myoglobin, particularly for the more disordered loop regions. Solvent accessibility measurements also showed some correlation with the UVPD fragmentation of holo-myoglobin. Comparison of UVPD of holo- and apo-myoglobin revealed similarities in fragmentation yields, particularly for the lower charge states (8 and 9+). Both holo- and apo-myoglobin exhibited low fragmentation yields for the AGH helical core, whereas regions known to interact with the heme show suppressed fragmentation for holo-myoglobin. The fragment yields from HCD showed the lowest correlation with B-factor values and rather reflected preferential charge-directed backbone cleavages.

 Please wait while we load your content...
Please wait while we load your content...