Dehydrogenation of formic acid by Ir–bisMETAMORPhos complexes: experimental and computational insight into the role of a cooperative ligand†
Abstract
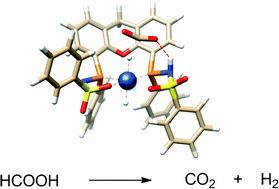
The synthesis and tautomeric nature of three xanthene-based bisMETAMORPhos ligands (La–Lc) is reported. Coordination of these bis(sulfonamidophosphines) to Ir(acac)(cod) initially leads to the formation of IrI(LH) species (1a), which convert via formal oxidative addition of the ligand to IrIII(L) monohydride complexes 2a–c. The rate for this step strongly depends on the ligand employed. IrIII complexes 2a–c were applied in the base-free dehydrogenation of formic acid, reaching turnover frequencies of 3090, 877 and 1791 h−1, respectively. The dual role of the ligand in the mechanism of the dehydrogenation reaction was studied by 1H and 31P NMR spectroscopy and DFT calculations. Besides functioning as an internal base, bisMETAMORPhos also assists in the pre-assembly of formic acid within the Ir-coordination sphere and aids in stabilizing the rate-determining transition state through hydrogen-bonding.

 Please wait while we load your content...
Please wait while we load your content...