New coordination features; a bridging pyridine and the forced shortest non-covalent distance between two CO32− species†
Abstract
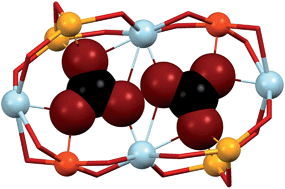
The aerobic reaction of the multidentate ligand 2,6-bis-(3-oxo-3-(2-hydroxyphenyl)-propionyl)-pyridine, H4L, with Co(II) salts in strong basic conditions produces the clusters [Co4(L)2(OH)(py)7]NO3 (1) and [Co8Na4(L)4(OH)2(CO3)2(py)10](BF4)2 (2). Analysis of their structure unveils unusual coordination features including a very rare bridging pyridine ligand or two trapped carbonate anions within one coordination cage, forced to stay at an extremely close distance (dO⋯O = 1.946 Å). This unprecedented non-bonding proximity represents a meeting point between long covalent interactions and “intermolecular” contacts. These original motifs have been analysed here through DFT calculations, which have yielded interaction energies and the reduced repulsion energy experimented by both CO32− anions when located in close proximity inside the coordination cage.

 Please wait while we load your content...
Please wait while we load your content...