Thermodynamics and kinetics of DNA nanotube polymerization from single-filament measurements†
Abstract
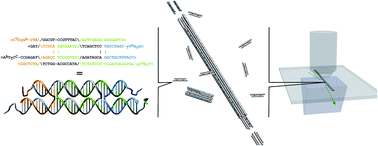
DNA nanotubes provide a programmable architecture for molecular self-assembly and can serve as model systems for one-dimensional biomolecular assemblies. While a variety of DNA nanotubes have been synthesized and employed as models for natural biopolymers, an extensive investigation of DNA nanotube kinetics and thermodynamics has been lacking. Using total internal reflection microscopy, DNA nanotube polymerization was monitored in real time at the single filament level over a wide range of free monomer concentrations and temperatures. The measured polymerization rates were subjected to a global nonlinear fit based on polymerization theory in order to simultaneously extract kinetic and thermodynamic parameters. For the DNA nanotubes used in this study, the association rate constant is (5.99 ± 0.15) × 105 M−1 s−1, the enthalpy is 87.9 ± 2.0 kcal mol−1, and the entropy is 0.252 ± 0.006 kcal mol−1 K−1. The qualitative and quantitative similarities between the kinetics of DNA nanotubes, actin filaments, and microtubules polymerization highlight the prospect of building complex dynamic systems from DNA molecules inspired by biological architecture.

 Please wait while we load your content...
Please wait while we load your content...