Effect of main chain conformation on thermosensitivity in elastin-like peptide-grafted polylysine†
Abstract
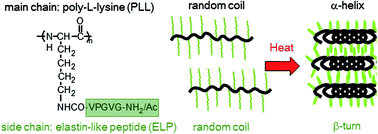
Stimuli-responsive polypeptides can be used in a wide variety of biomedical applications because of the biocompatibility. Elastin is a thermosensitive protein which contains unique repeat sequence such as VPGVG. Although short elastin-like peptides (ELPs) do not exhibit the temperature-dependent phase transition, grafting of ELP to a polymer scaffold provided the temperature-dependent properties. In this study, ELPs were conjugated to linear poly-L-lysine (PLL) and polylysine dendrimer (PLD) for the preparation of synthetic elastin-mimetic polypeptides. Polyallylamine and polyamidoamine dendrimers were also used as scaffolds of elastin-mimetic polymers and their thermosensitivity was compared. ELP-grafted PLL exhibited a lower phase transition temperature than ELP-grafted PLD, even though the molecular weight of ELP-grafted PLL was smaller. The conformations of the elastin-mimetic polymers changed from random coil to β-turn structures when they were heated. However, ELP-grafted PLL formed an α-helix when it was heated, and this effect was dependent on the pH. These results suggest that conformation changes of the main chains on the polypeptide affected their phase transition temperatures.

 Please wait while we load your content...
Please wait while we load your content...