Nitro-based selective inhibitors against matrix metalloproteinase-7 over matrix metalloproteinase-1†
Abstract
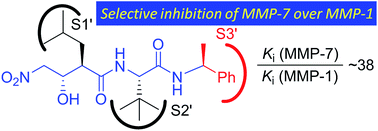
Matrix metalloproteinases (MMPs) are a family of zinc-dependent proteases, which catalyze the cleavage of the extracellular matrix and thus are tightly associated with various physiological and pathological processes. The MMP family contains at least 28 members, with only MMP-1, -2 and -7 experimentally validated as targets for cancer therapy. As reported in previous studies, the inhibition of MMP-1 could result in various side effects, including musculoskeletal syndrome, which should be avoided. However, “broad spectrum” inhibitors, which typically utilize hydroxamic acid as a strong zinc-binding group (ZBG), have been unapproved due to their lack of selectivity. Further, because both MMP-1 and -7 have similar S′1 hydrophobic pockets, it remains a challenge to explore the selective inhibition of MMP-7 over -1 up to now. In the presented paper, we firstly introduced a nitro group as a ZBG in the inhibition of MMP. A series of nitro-based dipeptidic compounds were thus synthesized and evaluated as MMP inhibitors. Combined with the kinetic assay results and computational analysis of the binding mode of the inhibitors with the active sites of the enzymes, it was disclosed that a reasonable adjustment of the P′3 side chains of the nitro-based MMP inhibitors could improve selectivity for the inhibition of MMP-7 over MMP-1.

 Please wait while we load your content...
Please wait while we load your content...