Formation of unusual dithiaphlorins from condensation of 2,5-bis(arylhydroxymethyl)thiophene and pyrrole†
Abstract
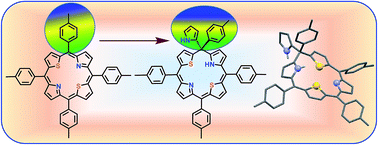
The first example of an unusual 21,23-dithiaphlorin containing pyrrole and aryl groups at the sp3 meso carbon that is present between the pyrrole and thiophene rings was isolated from the condensation of 2,5-bis(arylhydroxymethyl) and pyrrole under mild acid catalyzed conditions. The crystal structure revealed that the macrocycle is significantly distorted because of the presence of the sp3 meso carbon.

 Please wait while we load your content...
Please wait while we load your content...