Ionic liquid – containing ionogels by thiol–ene photopolymerization. Kinetics and solvent effect
Abstract
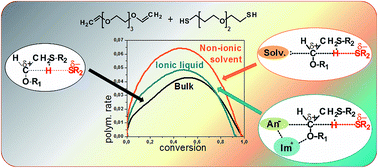
Photopolymerization of monomers in ionic liquids (ILs) leads to the formation of conducting ionogels. In the frame of our continuing investigations in this field we report the first example of thiol–ene polymerization in ILs. The work focuses on the polymerization kinetics and reaction mechanism. The model system is based on a difunctional thiol and a divinyl ether (to exclude ene homopolymerization) with spacers built from oxyethylene units. The photo-initiated polymerization was carried out in the presence of various amounts of imidazolium-based ILs and was compared to the polymerization in non-ionic solvents. It was found that the addition of the solvents to the investigated system accelerates the reaction; however, the reaction occurs faster in non-ionic solvents than in ILs. The accelerating effect is associated with the additional stabilization of a partial charge separation in the transition state and its magnitude is closely related to the Kamlet–Taft β parameter which describes the hydrogen bond accepting ability of the solvent. In the case of ILs, the stabilizing effect is exerted by the IL's anion. However, the imidazolium cation interacts with the ether oxygen of the monomer decreasing its stabilizing influence on the transition state (which is related to the Kamlet–Taft α parameter representing the hydrogen bond accepting ability). The knowledge of the presented relationships provides a tool for designing thiol–ene systems containing ILs.


 Please wait while we load your content...
Please wait while we load your content...